Videos
As part of the Forum’s efforts to raise awareness of biosimilars and provide evidence-based information regarding these medicines, we have created the following videos. If you would like to use the videos for your own educational purposes, please contact info@canadianbiosimilarsforum.ca
The Safety and Efficacy of Biosimilars This video outlines the safety of biosimilars, highlighting Health Canada’s statement that there are no clinically meaningful differences in the safety and efficacy of biosimilars and the reference biologic drugs.
Expanding Care with Biosimilars This video speaks to the billions of dollars in healthcare spending that could be saved with the use of biosimilars. These savings can be reinvested into the health system so that more patients can access more medicines.
Switching to a Biosimilar This video highlights Health Canada’s statement that a well-controlled switch from a reference biologic to a biosimilar is acceptable. A decision to switch should be discussed between a patient and their treating physician.
Infographics
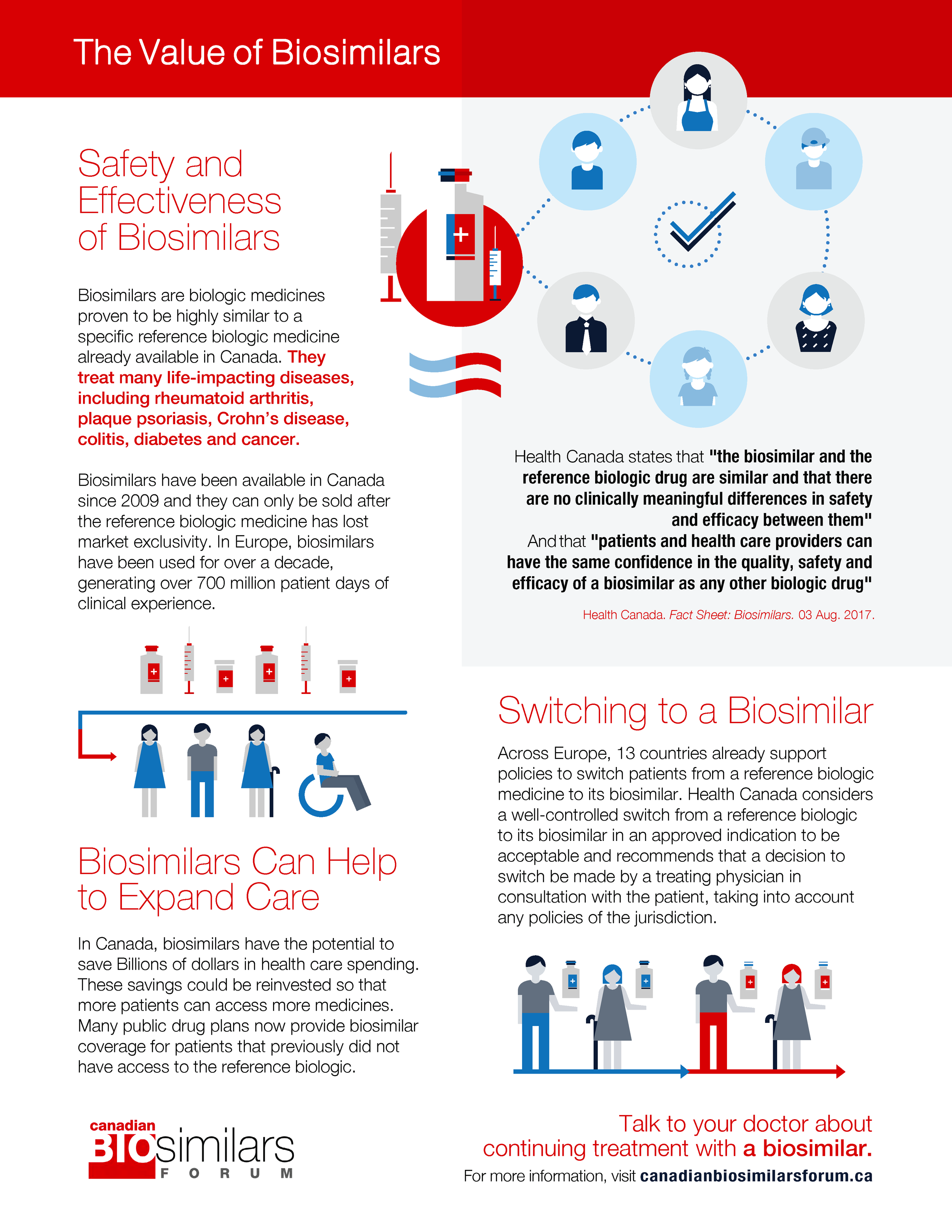
The Value of Biosimilars - Infographic This infographic outlines the points made from the three video resources above, namely: the safety and efficacy of biosimilars, their potential to expand care in the greater health system and the decision to switch.
Safety and Efficacy - Infographic This infographic introduces what biosimilars are used for and the countries and provinces in Canada that have adopted them.
Biosimilars can generate cost savings across Canada - Infographic This infographic explores the use of biosimilars in Canada, and the Biosimilars Initiative in British Columbia.
(NEW) Cost Savings Across Canada - Infographic This infographic outlines the cost savings of biosimilars from the PMPRB (Patented Medicine Prices Review Board) studies, and B.C. and Alberta’s biosimilars initiatives.
Key Links, Statements, and Reports
FROM OTHERS:
From Health Canada:
Health Canada Infographic for Patients
Health Canada Fact Sheet on Biosimilars
Health Canada – Biosimilars main page
Health Canada’s 2017 Biosimilars Workshop: Summary Report
Marketing of Biologics and Biosimilars
From the Canadian Agency for Drugs and Technologies in Health:
CADTH Background on Biosimilars for Patients
CADTH background on Biosimilars for Health Care Providers
From the University of Toronto:
From the Patented Medicine Prices Review Board:
PMPRB report on Potential Savings from Biosimilars in Canada
PMPRB report on Biologics in Canada - Market Trends Part 1
PMPRB report on Biologics in Canada - Market Trends Part 2: Biosimilar Savings
From the Ontario Drug Policy Research Network:
From Cancer Care Ontario:
Pan-Canadian Oncology Biosimilars Initiative
Oncology Biosimilars Resources for Providers
Other Canadian Resources:
ACE (Arthritis Consumer Experts) Joint Health
Arthritis Research Canada: Biosimilars - What you Need to Know
US Resources:
FDA Background on Biosimilars for Consumers
European Resources:
European Medicines Agency and European Commission report on Biosimilars in the EU